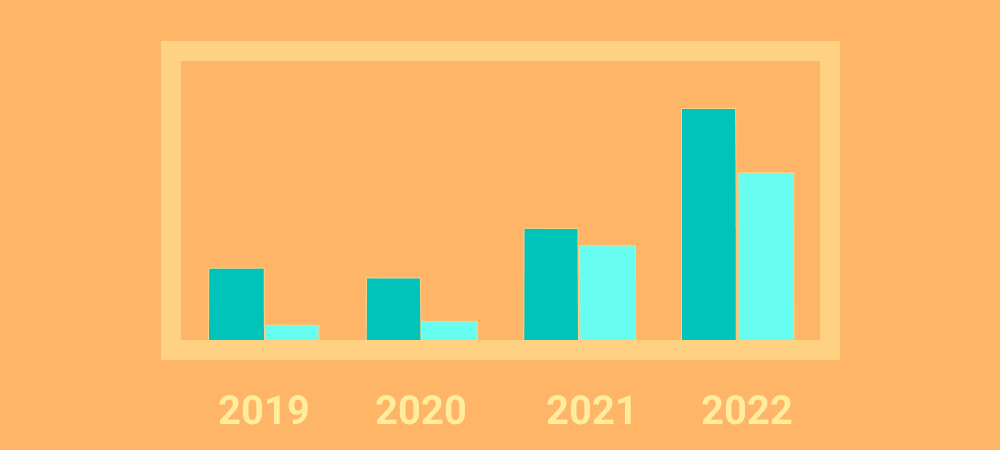
28 March 2023, Munich – Severe violations of Good Manufacturing Practice (GMP) resulting in FDA warning letters put additional strain on pharmaceutical supply chains, with a potentially compounding impact on drug shortages...To swiftly react or even predict non-compliance events like warning letters, companies need to be able to effectively and quickly analyze suppliers for their products, including their manufacturing sites. QYOBO and Qualifyze position their clients for success regarding non-compliance preparedness, by providing well-structured, up-to-date, high-quality inspection and audit data.