QYOBO integrates the first global database on drug shortages into its platform. The upgrade further expands its supply chain analytics to an end-to-end perspective – connecting marketing authorization (MA) holders to their active pharmaceutical ingredients (API) and key starting materials (KSM) manufacturers. Further additions include drug approvals and marketing authorizations from 45+ countries and detailed insights on 2,000+ finished dosage form (FDF) manufacturing sites.
“We’re setting a new industry standard, empowering stakeholders to proactively address drug shortages with unmatched precision and foresight. “
Dr. Markus Felgenhauer, Co-Founder & CEO of QYOBO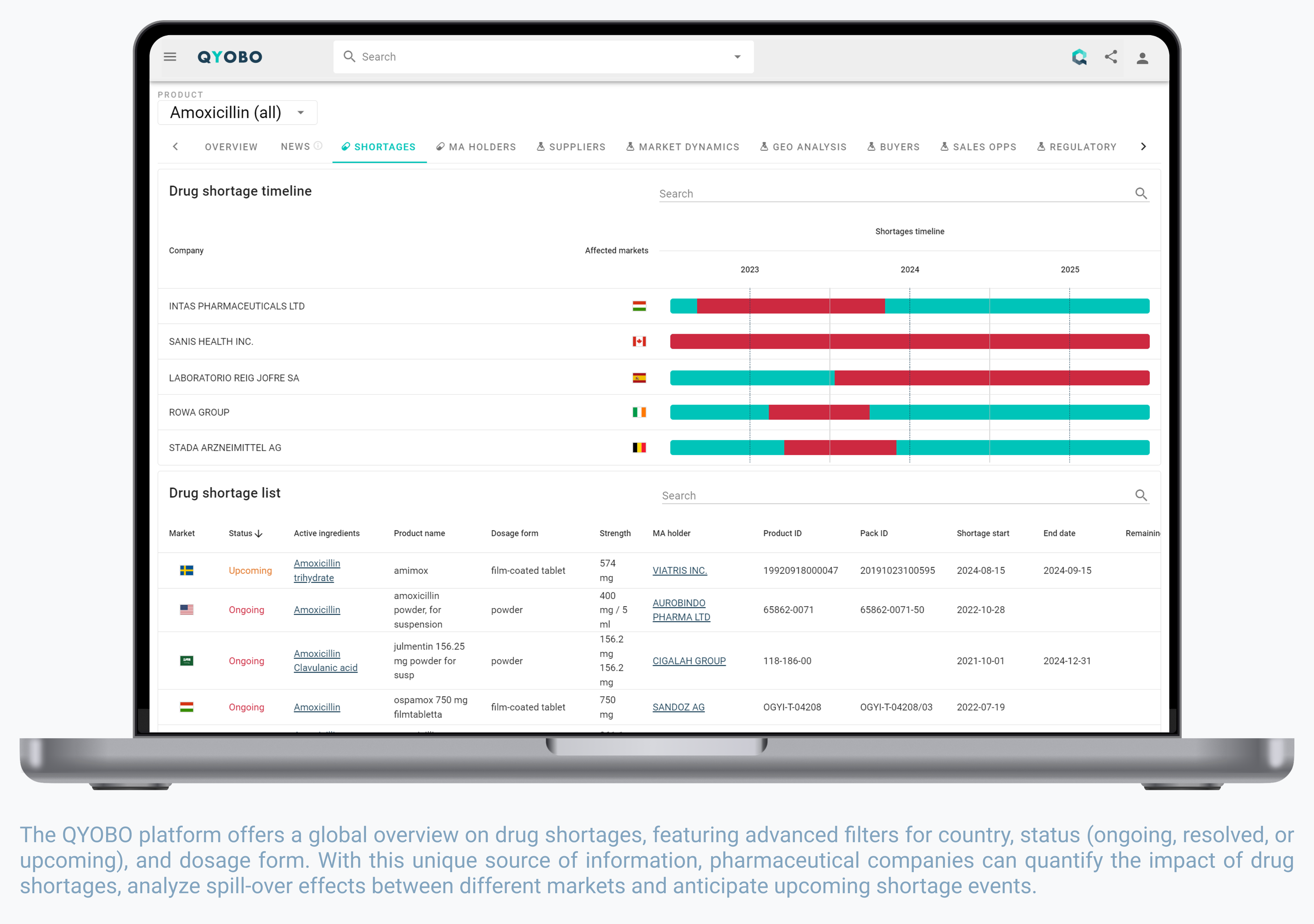
13 March 2024, Munich – QYOBO today announced a major upgrade to its platform introducing a global database on drug shortages and marketing authorizations, finished drug manufacturing sites, and end-to-end supply chain analytics. With this upgrade, QYOBO further underscores its commitment to providing the most powerful and actionable insights on pharmaceutical value chains. It addresses critical challenges such as global drug shortages that impact patients and healthcare systems worldwide.
Key features of the upgrade include:
- Global Drug Shortage Database: An industry-first, QYOBO introduces a global overview of drug shortages, covering resolved, ongoing, and upcoming shortages in 27 countries. Through the simultaneous addition of marketing authorizations, the QYOBO platform not only indicates products affected by shortages, but also remaining alternatives in the market. Combining this information in a single spot allows pharmaceutical companies to proactively prepare for upcoming events and identify weak links in the competitive landscape.
- Global Marketing Authorizations: Incorporating regulatory data from 45+ countries in 20+ languages, including China, the US, and Europe, the platform now also offers a consolidated view of worldwide marketing authorizations. This highlights where a company sells a specific product, or where gaps in drug availability and thus business opportunities exist, e.g., when few MA holders are offering a drug. The platform will share news on new marketing authorizations and discontinuations in Q2 this year.
- FDF Manufacturing Sites: Expanding our coverage on thousands of API manufacturing sites (covering both small molecule and biologics), the FDF module adds unique insights on 2,000+ finished drug manufacturing sites. Now, companies can easily identify alternative FDF manufacturing sites or benchmark their supplier base against the market. For each manufacturing site, the platform provides a detailed list of products manufactured there – providing an immediate impact assessment for non-compliance events such as warning letters.
- End-to-end Supply Chain Visibility: The vast amount of data elevates QYOBO’s supply chain analytics to the next level: Connecting MA holders to their FDF, API, and KSM manufacturers for precise back-tracing where a product for a specific market is manufactured. This empowers new use cases – from spotting weak links in the market and elevating tender responses to identifying which products and markets might be affected by non-compliance events such as warning letters. All of this is made possible by QYOBO’s ability to combine supply chain fragments in hundreds of global databases into one big picture – now with site-level accuracy.
 With 5+ million data sets on marketing authorizations and 200,000+ on drug shortages being processed daily, the QYOBO platform is set to become an indispensable tool for decision-makers in the pharmaceutical industry. Offering unique, actionable insights along the entire pharmaceutical value chain, QYOBO sets a new industry standard – driving proactive action to avert drug shortages and boost the availability of essential medicines worldwide.
With 5+ million data sets on marketing authorizations and 200,000+ on drug shortages being processed daily, the QYOBO platform is set to become an indispensable tool for decision-makers in the pharmaceutical industry. Offering unique, actionable insights along the entire pharmaceutical value chain, QYOBO sets a new industry standard – driving proactive action to avert drug shortages and boost the availability of essential medicines worldwide.
About:
QYOBO is a Munich-based big data startup specializing in pharmaceutical market intelligence. Its mission is to improve global healthcare by providing critical insights into the pharmaceutical supply network and transforming complex data into actionable intelligence. Providing unique information on supply chains, manufacturing sites, market prices, and more, in a single platform, QYOBO empowers decision-makers and their teams to make better decisions in a fraction of the time.
Press contact:
Dr. Sandra Fill
QYOBO GmbH | Marienplatz 28 | 80331 Munich
+49 151 19790466 | sandra@qyobo.com
linkedin.com/company/qyobo