31 August 2023, Munich – The average global price (dark blue line) of semaglutide API decreased by 72% (Figure 1) over the last five years, from 3,000K USD/kg to 843K USD/kg. The most competitive prices (“lower 20% on the QYOBO platform represented as a green line) within the market reached a record low of 134K USD/kg in 2023.
This strong downward dynamic can be explained by the increasing number of market participants, and hence increased competition, and also larger volumes which generally bring economies of scale.
The API price, however, is just one element of the drug manufacturing cost. Currently, three FDA-approved semaglutide drugs are on the market – Ozempic, Rybelsus, and Wegovy. The first two – Ozempic injection and Rybelsus tablet – are two different drugs to treat type 2 diabetes by lowering the level of blood sugar, with average expenses of more than 900 USD per month in the US. The latter – Wegovy injection – is prescribed to help patients with obesity and weight-related medical issues. It contains higher doses of semaglutide and is therefore even more costly (more than 1,300 USD in the US per month).1 2
For each treatment, Figure 2 summarizes the frequency, annual API dosage, and calculated annual API manufacturing cost for a single patient.3
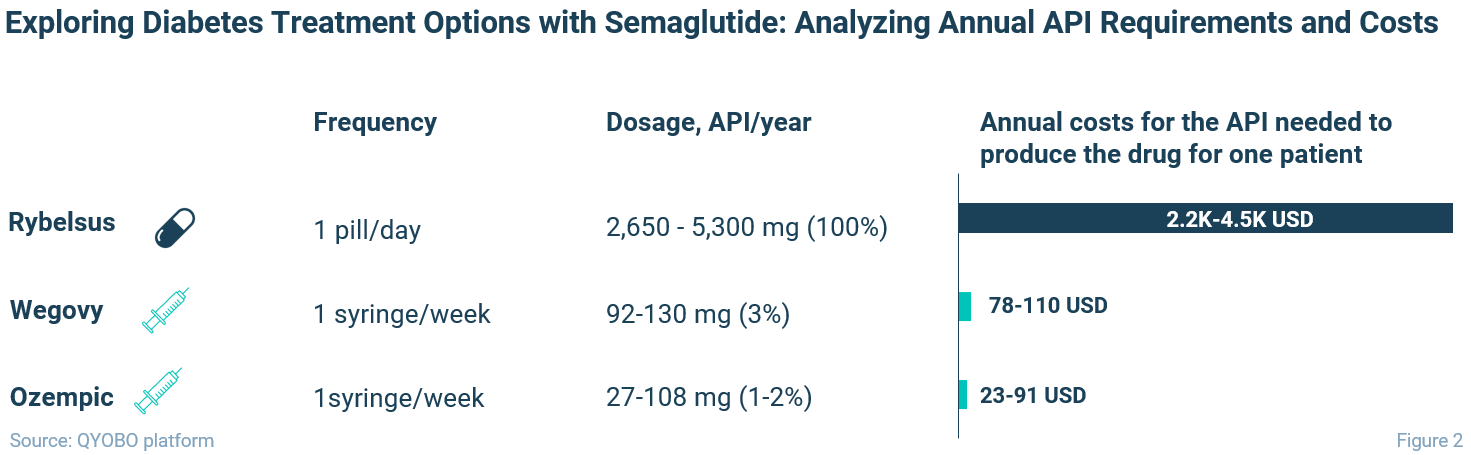
Obviously, the tablet form of the drug (Rybelsus) requires a much larger API quantity than its injectable counterparts. Given that despite the falling price curve, the API cost remains relatively high, tablet manufacturers of generic versions of Rybelsus will face substantially higher API expenses. Furthermore, considering the current shortage of the API raw material, the availability of tablet versions may be limited.
Extremely high dosing for the oral dosage form of semaglutide is necessary due to its excessively low bioavailability (<1% for Rybelsus) 4, meaning that the amount of the API required for supplying one person with this medication is the same as for over 100 patients treated with Ozempic. Nonetheless, the commercial success of Rybelsus proves that people favor the option of oral treatment, which is not surprising: the form of the pill makes the drug more appealing to patients who dislike needles due to their inconvenience and discomfort. 5 6 7
Innovative new technologies might be able to bring the best of both worlds – the ease of use of a tablet and the low API dosage of an injection – together: A prime example is the American company Rani Therapeutics, which developed a mechanical method for oral delivery of biologics, the “RaniPill” (see Figure 3). It is designed to deliver any biologic drug directly through the intestinal wall via a dissolvable microneedle. The capsule is protected by a special pH-sensitive polymer coating, which ensures the pill arrives in the small intestine without first dissolving in the stomach. Once arrived at its destination, the capsule dissolves, and a small balloon inflates, driven by a chemical reaction. The pressure inside the balloon eventually pushes a small needle into the intestinal wall, where the needle itself dissolves, thereby delivering the drug payload.
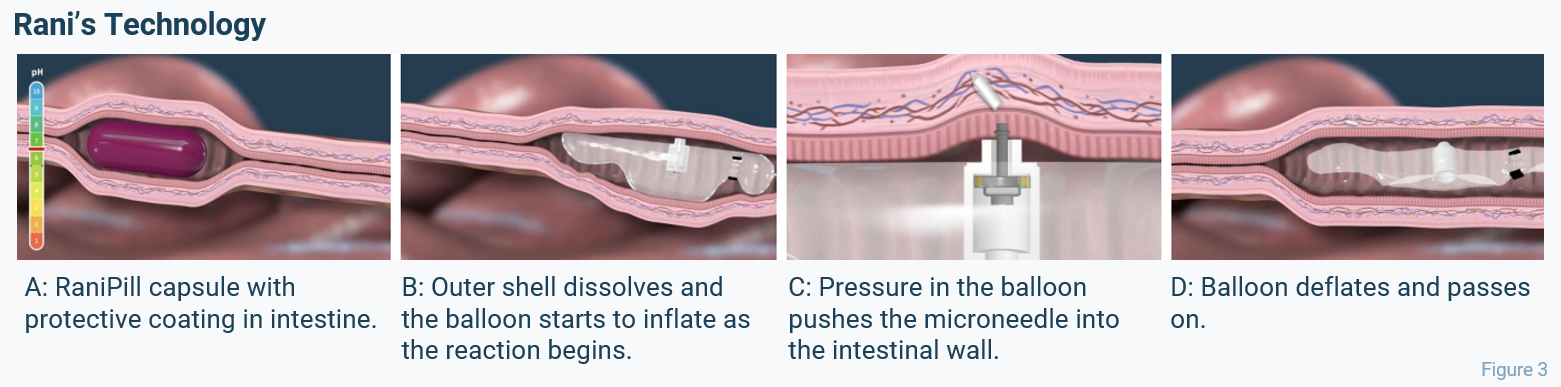
Because the RaniPill delivers the drug payload with bioavailability similar to subcutaneous injections, it requires less API in the manufacturing process compared to current oral formulations. Considering the shortage situation for semaglutide – which, as for most biologics – is largely driven by limitations to rapidly expand up API manufacturing capacity to keep up with demand, this kind of delivery method can contribute to making the treatment available to a larger patient population while potentially also reducing overall drug manufacturing cost. In an interview, the team at Rani Therapeutics also shared that their product enables optimum titration of drug serum levels which enables tighter therapeutic banding and has the potential for better clinical efficacy.
For further details on the RaniPill, reach out to the contact mentioned below. For more detailed market analytics on Semaglutide, contact the QYOBO sales team instead.
About QYOBO GmbH
QYOBO’s mission is to improve access to essential medication for everyone by contributing to a more transparent, efficient and robust supply of pharmaceutical and chemical raw materials.
For this purpose, we’ve developed the QYOBO market analytics platform for APIs, intermediates and chemicals. From millions of trade, regulatory and financial datasets scattered around the world, our big data algorithms derive unique, actionable insights on market prices and trends, suggest suitable partners for your business and automate data-heavy workflows in procurement, supply chain and business development.
Founded in June 2019 and based in Munich, our company is pursuing its mission collaboratively with its international clients and has been recognized with numerous awards including the BASF market challenge and the Digital Innovation award 2020 by the German Federal Ministry for Economic Affairs & Energy (BMWi).
About Rani Therapeutics
Rani Therapeutics is a clinical stage biotech company that has developed a platform technology to enable oral delivery of biologic drugs.
Millions of patients with chronic conditions require biologic drugs, the vast majority of which must be injected. We have developed the RaniPill™ technology to replace subcutaneous or IV injections of biologics with an oral pill. The RaniPill™ capsule is designed to deliver an injection to the intestinal wall, where there are no sharp pain receptors. Delivery via the RaniPill has achieved bioavailability similar to subcutaneous injections across a multitude of molecules including peptides, monoclonal antibodies, large proteins, and hormones. Rani plans to conduct our first Phase 2 clinical study beginning in 2023.
At Rani, we are working with world class experts and innovators to achieve our mission of ending the burden of painful injections for millions of patients.
Disclaimer:
The information in this article is not intended to be used for medication purposes. Please do not self-medicate and consult a physician/doctor for any questions with regard to your personal medical needs. This assessment has been prepared adhering to the highest quality standards based on a variety of external data sources (see sources) with the purpose of making distributed information accessible to a broader audience. The information contained in this document is provided on an “as is” basis and QYOBO GmbH assumes no responsibility or liability for the completeness, accuracy, usefulness or timeliness of the information provided. This article contains links to external websites operated by third parties upon which QYOBO GmbH has no influence. QYOBO GmbH does not assume any guarantee or liability for third party content.
² Fiercepharma, 18.08.2023.
List prices for Ozempic, Wegovy far higher in the
US than in peer nations: KFF
5 Fiercepharma, 02.02.2023.
Novo’s Rybelsus comes into its own as Wegovy shakes off supply constraints
6 Pharmaceutical Technology, 12.02.2021.
7 Abcnews, 26.06.2023
No more needles? A daily pill may work as well as Wegovy shots to treat obesity
For further information please contact:
Ms. Iuliia Voronina,
Corporate Communications,
QYOBO GmbH