11 October 2023, Munich – In previous articles, we often addressed the prevalent issue of drug shortages, which made waves in 2022 and continued gaining momentum in 2023. Developing our upcoming “Drug shortage module” for the QYOBO platform, we learned that it’s usually a mix of reasons causing major disruptions. One of these reasons is the violation of Good Manufacturing Practice (GMP). Severe violations discovered during an audit by the U.S. FDA can result in a warning letter, one of the most impactful instruments at the FDA’s disposal, as – among other restrictions – it hinders the company from launching new products developed at this site. In the previous year alone, the U.S. FDA issued 13 warning letters to major companies.

In 2023, 5 warning letters were issued so far. Warning letters can affect different parts of the business depending on the observations made during the FDA inspections. In some cases, the impact prevents the site from delivering products to global markets for years. Others have mild or limited ramifications on global supply chains. In any event, pharmaceutical companies are often struggling for weeks to determine whether they are affected and how it will impact their business – as it is difficult to identify which products are made at a particular site.
A recent example is the Indian company Centaur Pharmaceuticals Private Ltd., which received two warning letters this summer – one for its API manufacturing site Centaur Pharmaceuticals Private Ltd. (Chikhloli, Ambernath) and the other for its FDF manufacturing site Centaur Pharmaceuticals Private Ltd. (Pune). For any manufacturing site, the QYOBO platform provides a neat overview of relevant news about the site, GMP inspection history, as well as further details on the products manufactured at this location. The latter is shown in Figure 1 – for Centaur’s Chikhloli API manufacturing site. In total, the QYOBO platform highlights 19 APIs that are manufactured at this site. Now an obvious question is – who is using these APIs in their finished products?
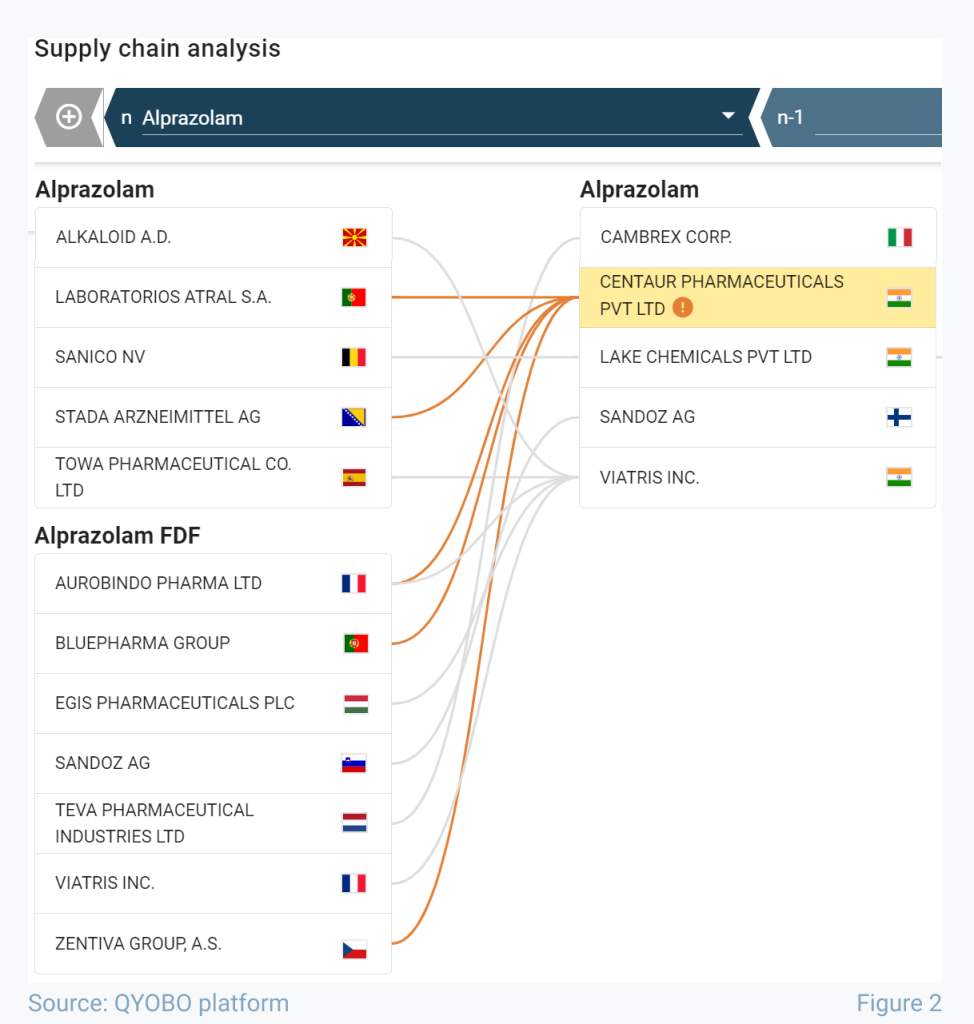
For that, the supply chain module of the QYOBO platform provides further insights. It shows an instant overview of which companies are working with a particular supplier, thus empowering QYOBO users to access their own supply risks as well as estimate the potential impact of non-compliant events on the pharmaceutical supply network as a whole. Figure 2 illustrates the example of the supply chain of alprazolam, one of the 19 APIs manufactured in Chikhloli. The view is filtered to highlight 12 key European companies purchasing this API*. In the supply chain module, sites with recent warning letters are immediately highlighted, and orange connections emphasize which companies are using the products manufactured at this location. In this case, more than 40% of alprazolam buyers are sourcing from Centaur, and what is more, most of them appear to be single-sourced.
The example offered above is all but a simple showcase of what pharmaceutical organizations can obtain within seconds when the vast amount of public market and regulatory data is thoroughly structured and turned into actionable insights. For further use cases, e.g., related to mitigating drug shortages or spotting supply chain risks early with our compliance score, please feel free to contact our colleagues mentioned below.
* Filtered to show European companies only. To obtain the full list of affected companies feel free to reach out to the QYOBO sales team
About QYOBO GmbH
QYOBO’s mission is to improve access to essential medication for everyone by contributing to a more transparent, efficient and robust supply of pharmaceutical and chemical raw materials.
For this purpose, we’ve developed the QYOBO market analytics platform for APIs, intermediates and chemicals. From millions of trade, regulatory and financial datasets scattered around the world, our big data algorithms derive unique, actionable insights on market prices and trends, suggest suitable partners for your business and automate data-heavy workflows in procurement, supply chain and business development.
Founded in June 2019 and based in Munich, our company is pursuing its mission collaboratively with its international clients and has been recognized with numerous awards including the BASF market challenge and the Digital Innovation award 2020 by the German Federal Ministry for Economic Affairs & Energy (BMWi).
Disclaimer:
The information in this article is not intended to be used for medication purposes. Please do not self-medicate and consult a physician/doctor for any questions with regard to your personal medical needs. This assessment has been prepared adhering to the highest quality standards based on a variety of external data sources (see sources) with the purpose of making distributed information accessible to a broader audience. The information contained in this document is provided on an “as is” basis and QYOBO GmbH assumes no responsibility or liability for the completeness, accuracy, usefulness or timeliness of the information provided. This article contains links to external websites operated by third parties upon which QYOBO GmbH has no influence. QYOBO GmbH does not assume any guarantee or liability for third party content.
For further information please contact:
Ms. Iuliia Voronina,
Corporate Communications,
QYOBO GmbH