8 February 2023, Munich – A rise in severe cases of infections caused by Strep A, with several children dying from the disease, was announced this winter by the European Center for Disease Prevention and Control and the World Health Organization. The antibiotic amoxicillin – used for treating Strep A as well as other infections of the respiratory and urinary tract and skin – is now in short supply. Moreover, according to the European Medicines Agency, a gap in antibiotics supply across the EU is a chronic phenomenon, and currently 25 of the 27 member states report active shortages for these drugs.1 A similar supply situation is experienced in the United States as a demand
increase for amoxicillin has led to a nationwide shortage. The problem is further escalated by manufacturing constraints and the consequences of the pandemic as well as the energy crisis in Europe, according to a spokesman of Sandoz, a key manufacturer of amoxicillin.2 3
In turbulent times like these, it’s essential for companies to have immediate and up-to-date access to information on the global supply network in order to react swiftly or prevent further supply disruptions. To support our clients in this effort, we launched a supply chain module on the QYOBO platform last year. Based on trade and regulatory data from hundreds of databases worldwide, it provides critical information about supply chains and even pin-points the locations of manufacturing sites (see figure 1 for amoxicillin trihydrate).
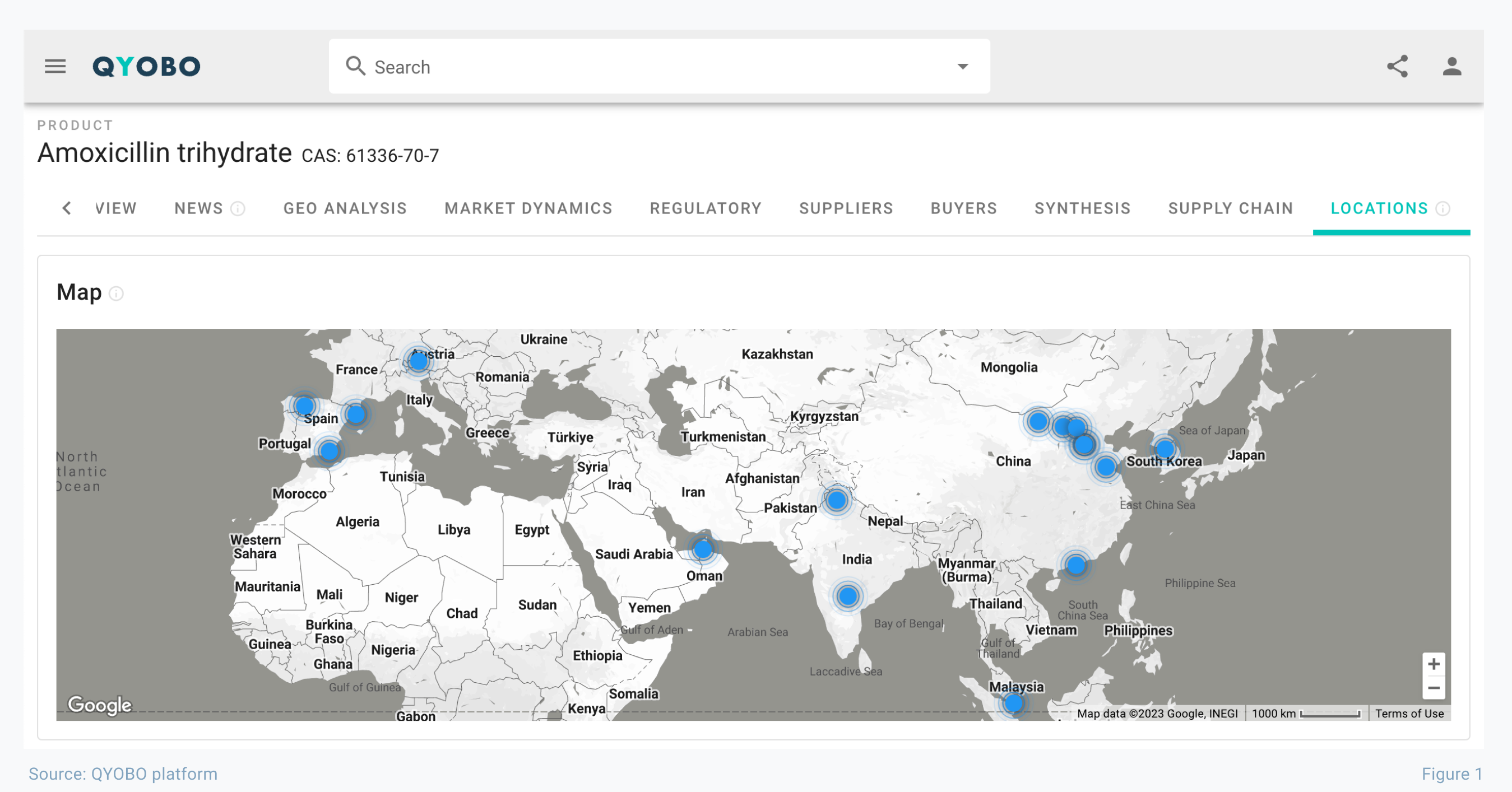
While some amoxicillin manufacturers remain in Europe, the majority of the production is now predominantly in Asia, most notably India and China. The dependence of the global supply on these countries over the past decades has led to a considerable geopolitical challenge with frequent political discussions on EU level 4, EU national level 5 6 7, and in the US congress. 8
More common than geopolitical incidents, however, are technical defects or non-compliance events impacting global supply chains. In the latter case, e.g. when a warning letter is issued to a manufacturing site, the effects ripple throughout the entire industry. Due to the lack of easily accessible public data, gauging the impact of such an event on drug availability and thus patients has historically been difficult to access.
Powered by millions of datasets, the supply chain module of the QYOBO platform provides all this information in an instant – connecting manufacturing sites, regulatory news (such as non-compliance events), and global supply chain insights in a single view.
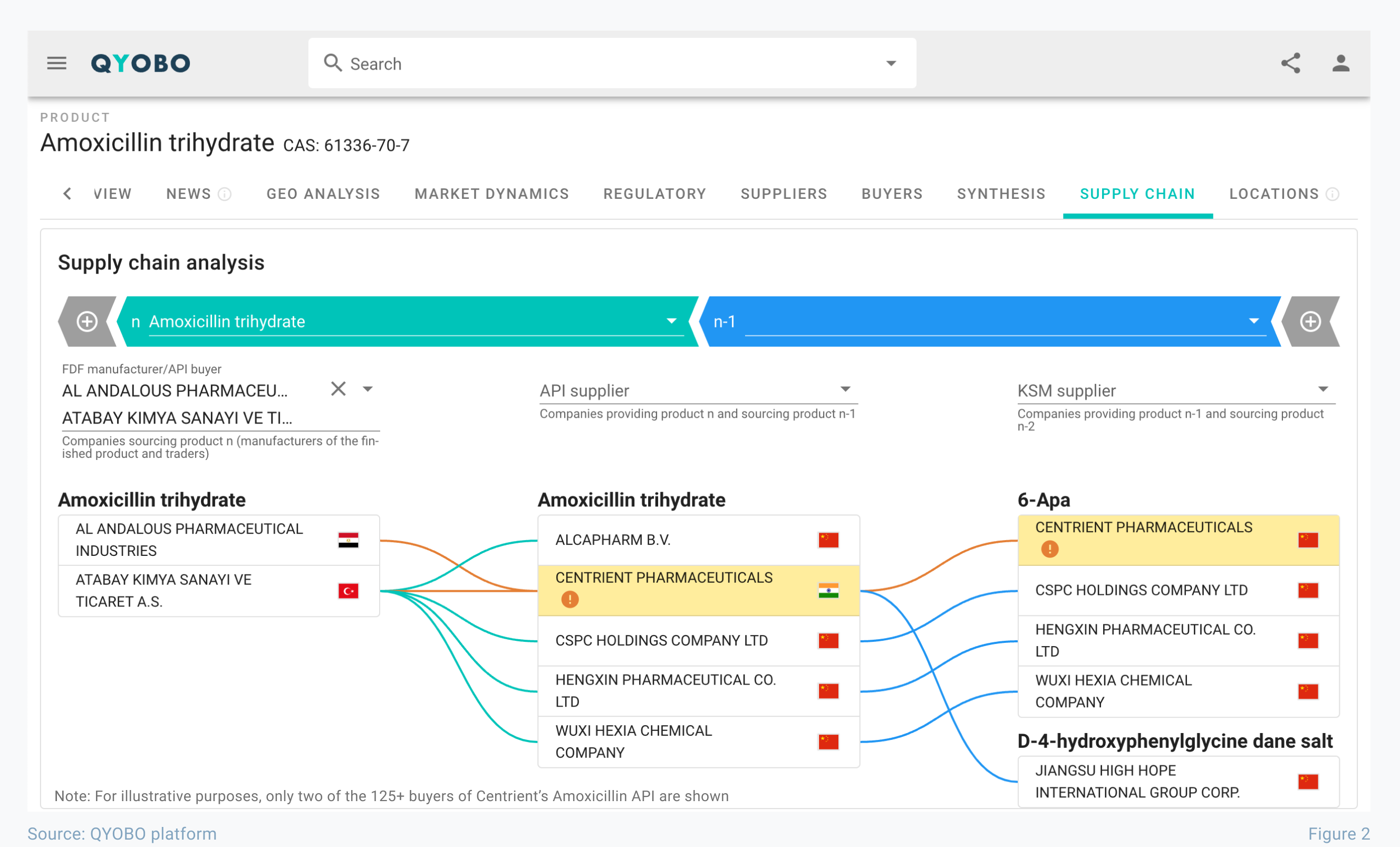
An example for amoxicillin trihydrate is provided in figure 2: Centrient Pharmaceuticals, one of the largest API manufacturers for the substance, received a warning letter in early December (accessible on the QYOBO platform on the same day). Based on our insights for manufacturing locations, the QYOBO platform identified Amoxicillin trihydrate as one of several APIs that are manufactured at this location, immediately highlighting the event in the supply chain module. The impact is significant – as more than 125 companies around the globe rely on the API produced at this manufacturing site.
Apart from enabling clients to easily assess whether they are affected by any given event and making it possible for governments to evaluate the overall impact on supply continuity, the supply chain module also provides competitive insights. For example, by benchmarking the resilience of your own supply chain against competitors (see figure 3). In times of volatility, assessing whether competitors are double-sourced or at risk of supply disruptions provides the opportunity to increase market shares while contributing to higher drug availability for patients.

Figure 3 illustrates these use cases – leaning on the above mentioned warning letter. The two companies selected on the very left – Atabay Kimya Sanayi Ve Ticaret A.S. and Al Andalous Pharmaceutical Industries – are FDF manufacturers and both clients of Centrient Pharmaceuticals. A closer look at the two companies shows stark differences in their supply chains: Atabay Kimya Sanayi Ve Ticaret A.S. is working with several sources and thus can fall back on alternative suppliers. In contrast – based on all available information – Al Andalous Pharmaceutical Industries seems to rely on Centrient Pharmaceuticals as sole supplier for this API.
In summary, “big supply chain data” turns previously impossible use cases into reality. This empowers pharmaceutical companies to rethink how they manage and monitor their own supply chains as well as identify new business opportunities. For further details or a demo of the QYOBO platform, feel free to reach out to sales@qyobo.com.
Glossary
Active Pharmaceutical Ingredient
Key Starting Material
About QYOBO GmbH
QYOBO’s mission is to improve access to essential medication for everyone by contributing to a more transparent, efficient and robust supply of pharmaceutical and chemical raw materials.
For this purpose, we’ve developed the QYOBO market analytics platform for APIs, intermediates and chemicals. From millions of trade, regulatory and financial datasets scattered around the world, our big data algorithms derive unique, actionable insights on market prices and trends, suggest suitable partners for your business and automate data-heavy workflows in procurement, supply chain and business development.
Founded in June 2019 and based in Munich, our company is pursuing its mission collaboratively with its international clients and has been recognized with numerous awards including the BASF market challenge and the Digital Innovation award 2020 by the German Federal Ministry for Economic Affairs & Energy (BMWi).
For further information please contact:
Ms. Iuliia Voronina, Corporate Communications
communication@qyobo.com
www.qyobo.com
Disclaimer: The information in this article is not intended to be used for medication purposes. Please do not self-medicate and consult a physician/doctor for any questions with regard to your personal medical needs. This assessment has been prepared adhering to the highest quality standards based on a variety of external data sources (see sources) with the purpose of making distributed information accessible to a broader audience. The information contained in this document is provided on an “as is” basis and QYOBO GmbH assumes no responsibility or liability for the completeness, accuracy, usefulness or timeliness of the information provided. This article contains links to external websites operated by third parties upon which QYOBO GmbH has no influence. QYOBO GmbH does not assume any guarantee or liability for third party content.
¹ Politico, 16.12.2022.
² Bloomberg, 02.10.2022.
5 Wirtschaftswoche, 02.12.2022.
Papier: Bundeswirtschaftsministerium will Regeln für China-Geschäft verschärfen
6 Die Bundesregierung, 2021.
Koalitionsvertrag zwischen SPD, Bündnis 90/Die Grünen und FDP