28 July 2021, Munich – When Dr. Baruch Bloomberg discovered the hepatitis B virus in 1965, he might not have assumed how many lives he would save in the future by developing a diagnostic test and vaccine against the virus. To honor Dr. Bloomberg’s remarkable work, the World Health Organization (WHO) declared his birthday on July 28 as World Hepatitis Day to increase awareness of the disease.¹ Being one of the most potent medications to treat hepatitis B, entecavir was approved in 2005 by the FDA for medical use and has been on WHO’s list of essential medicines ever since.²

On the occasion of World Hepatitis Day, QYOBO took a closer look into the supply chain for entecavir, highlighting a novel functionality of the QYOBO platform. As one of the most common medications to treat hepatitis B, a stable supply chain from the key starting materials (KSM aka as intermediates such as 2-Amino-6-chloropurine in the case of entecavir), to the active pharmaceutical ingredient (API) entecavir and finally to the finished drug is essential.
Starting with drug manufacturers, companies like German STADA Arzneimittel AG or Thailandese Pharmaland Group have a variety of suppliers that can be found mostly in Asia (e.g. Zhejiang Ausun Pharmaceutical Co., Ltd from China, EstechPharma Co., Ltd. from South Korea, Cadila Healthcare Ltd. from India or Orgchem Technologies, Inc. from Taiwan) with additional sites in Switzerland (Siegfried AG), Canada (Eurofins Group) and Ireland (SK Chemicals Co., Ltd). (see figure 1)
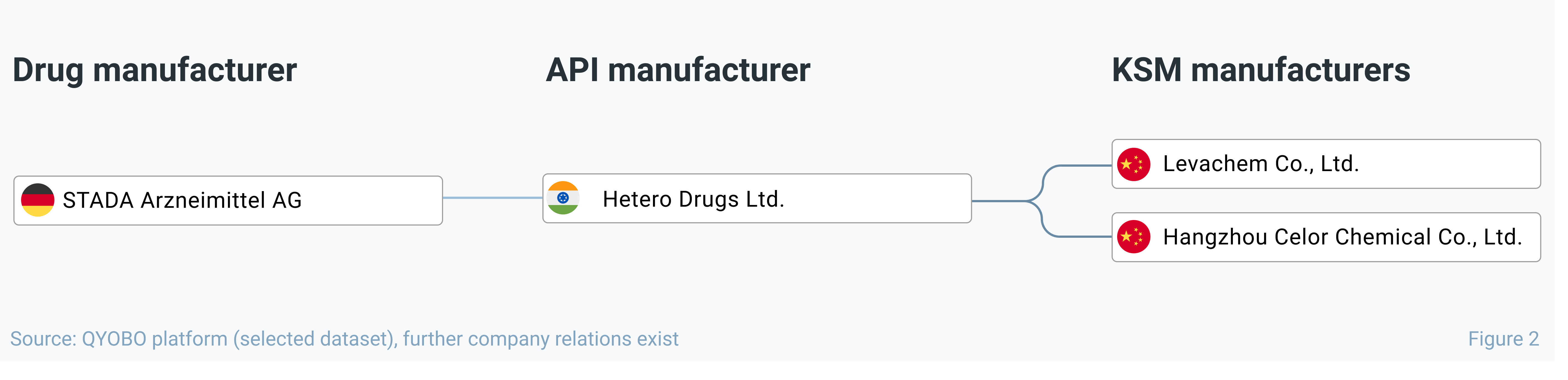
Using STADA as an example, figure 2 dives deeper into its entecavir supply chain with Indian Hetero Drugs Ltd. as its single (publicly known) API manufacturer which in turn is double-sourced from two Chinese KSM manufacturers from. Hence, while apparently single-sourced on the API-level, STADA’s supply chain is double-sourced for on the KSM-level providing moderate resilience upstream in the supply chain.
With this in mind, QYOBO aims to help its clients understand global supply chains and identify potential risks along the entire value chain from the base chemicals or intermediates to the API and finally the finished drugs. As such, the QYOBO platform gives pharmaceutical and chemical companies unprecedented opportunities to shore up supply chains by identifying risks in advance and providing alternatives when an incident occurs.
About QYOBO GmbH
QYOBO’s mission is to improve access to essential medication for everyone by contributing to a more transparent, efficient and robust supply of pharmaceutical and chemical raw materials.
For this purpose, we’ve developed the QYOBO market analytics platform for APIs, intermediates and chemicals. From millions of trade, regulatory and financial datasets scattered around the world, our big data algorithms derive unique, actionable insights on market prices and trends, suggest suitable partners for your business and automate data-heavy workflows in procurement, supply chain and business development.
Founded in June 2019 and based in Munich, our company is pursuing its mission collaboratively with its international clients and has been recognized with numerous awards including the BASF market challenge and the Digital Innovation award 2020 by the German Federal Ministry for Economic Affairs & Energy (BMWi).
For further information please contact:
Ms. Thuy Linh Nguyen, Corporate Communications
communication@qyobo.com
www.qyobo.com
Disclaimer: The information in this article is not intended to be used for medication purposes. Please do not self-medicate and consult a physician/doctor for any questions with regard to your personal medical needs. This assessment has been prepared adhering to the highest quality standards based on a variety of external data sources (see sources) with the purpose of making distributed information accessible to a broader audience. The information contained in this document is provided on an “as is” basis and QYOBO GmbH assumes no responsibility or liability for the completeness, accuracy, usefulness or timeliness of the information provided. This article contains links to external websites operated by third parties upon which QYOBO GmbH has no influence. QYOBO GmbH does not assume any guarantee or liability for third party content.
¹ World Health Organization, 27 July 2020.
Hepatitis B
² World Health Organization.
Model list of essential medicines