22 March 2020, Munich
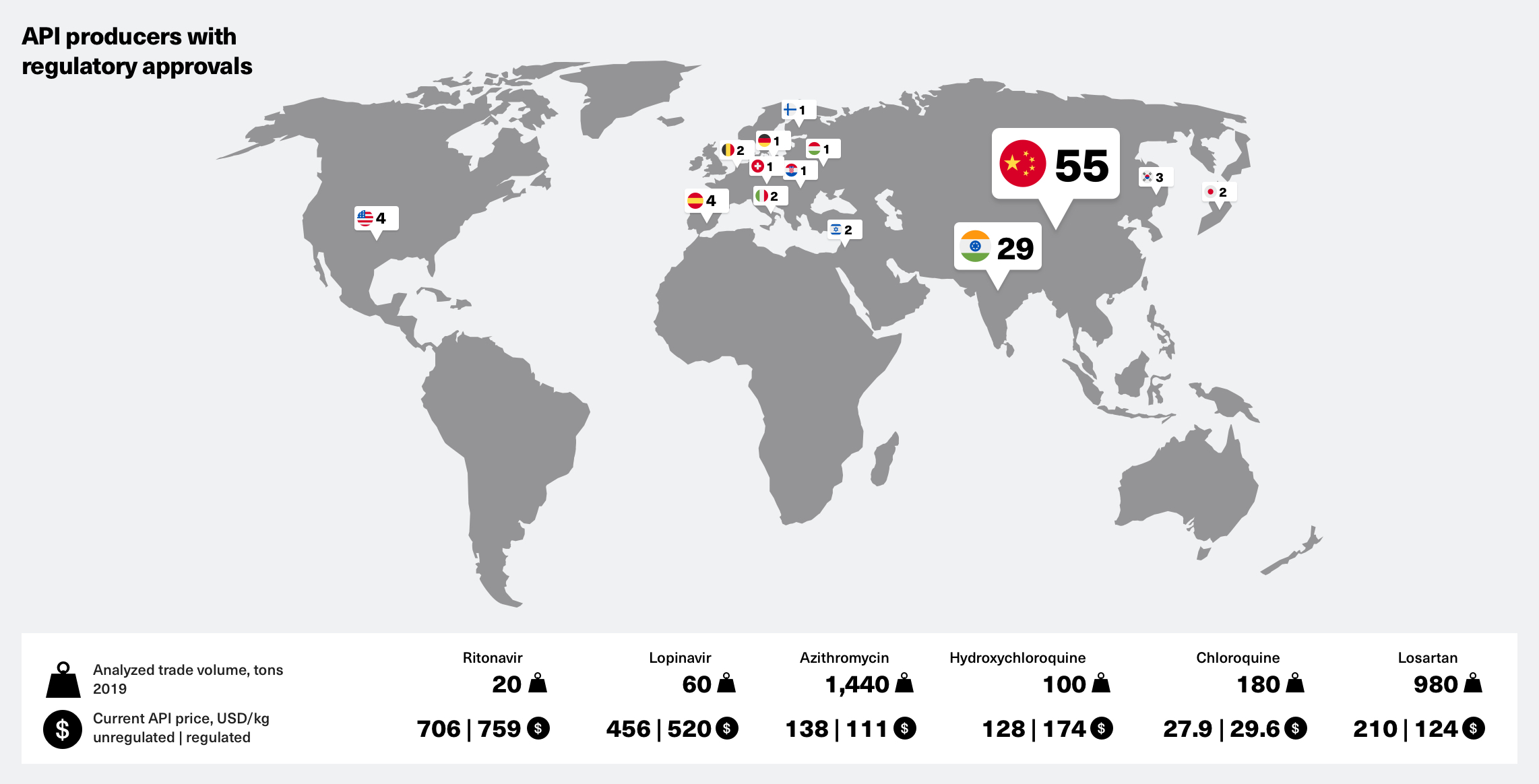
API producers with regulatory approvals
 CEP
CEP  USDMF
USDMF  JDMF
JDMF  KDMF
KDMF  CN-REG
CN-REG
- Various drugs are under evaluation against CoViD-19 by regulatory authorities worldwide
- The medications that recently have received particular attention are mainly used against:
• Malaria – Chloroquine/Hydroxychloroquine
• HIV – Lopinavir & Ritonavir
• High blood pressure – Losartan
• Bacterial infections – Azithromycin - If these prove effective in coronavirus trials, finished drug manufacturers would require larger amounts of these active ingredients (APIs) meet the increased demand
- Some API producers are already ramping up production, but it may not be enough to prevent supply chain disruptions and resulting drug shortages
- To help finished drug manufacturers evaluate all options for their supply chain – please find a worldwide overview of API producers with valid regulatory approvals below
Sources: QYOBO platform; United States Food and Drug Administration (FDA); European Medicines Agency (EMA); Pharmaceuticals and Medical Devices Agency (PMDA), Japan; National Institute of Food and Drug Safety Evaluation (NIFDS), South Korea; National Medical Products Administration (NMPA), China; company websites and recent announcements by company representatives; international trade statistics.
Disclaimer:
The information in this article is not intended to be used for medication purposes. It is explicitly stated, that the above substances are still UNDER EVALUATION and have not yet been formally approved by the relevant regulatory authorities (e.g. WHO, EMA, FDA) for treatment of CoViD-19. Please do not self-medicate and consult a physician/doctor for any questions with regard to your personal medical needs. This assessment has been prepared adhering to the highest quality standards based on a variety of external data sources (see sources) with the purpose of making distributed information accessible to a broader audience.
The information contained in this document is provided on an “as is” basis and QYOBO GmbH assumes no responsibility or liability for the completeness, accuracy, usefulness or timeliness of the information provided. The ability of some companies to provide goods may be limited in some countries or regions limited by intellectual property rights, e.g. patents. Companies without publicly available website were excluded in this assessment. QYOBO GmbH is a privately held tech startup headquartered in Munich, Germany. None of the companies mentioned in this analysis were involved in this analysis or hold control (shares) in QYOBO GmbH. This article contains links to external websites operated by third parties upon which QYOBO GmbH has no influence. QYOBO GmbH does not assume any guarantee or liability for third party content.