17 August 2021, Munich – In 2018, there was a shortage of life-saving blood pressure drugs for several months following a voluntary recall announcement by the FDA for medical products containing valsartan. The Active Pharmaceutical Ingredient (API) was contaminated with N-nitrosodimethylamine (NDMA), which could cause cancer and was detected during laboratory tests.1 As a result, many major pharmaceutical companies recalled batches of their medications containing valsartan from Chinese manufacturer Zhejiang Huahai Pharmaceuticals, the key manufacturer of sartans at the time. Now in August 2021, there are similar incidents like the Spanish Agency for Medicines and Health Products (AEMPS)2,3,4 reported the withdrawal of 120 different batches of medication containing valsartan and irbesartan also caused by NDMA impurities.5
Another incident occurred in May this year, when Teva and Bristol Laboratories voluntarily recalled drugs containing losartan potassium and irbesartan quoting similar NMDA impurities.6 The increasing number of recalls has already affected the raw material market for losartan potassium (figure 1).
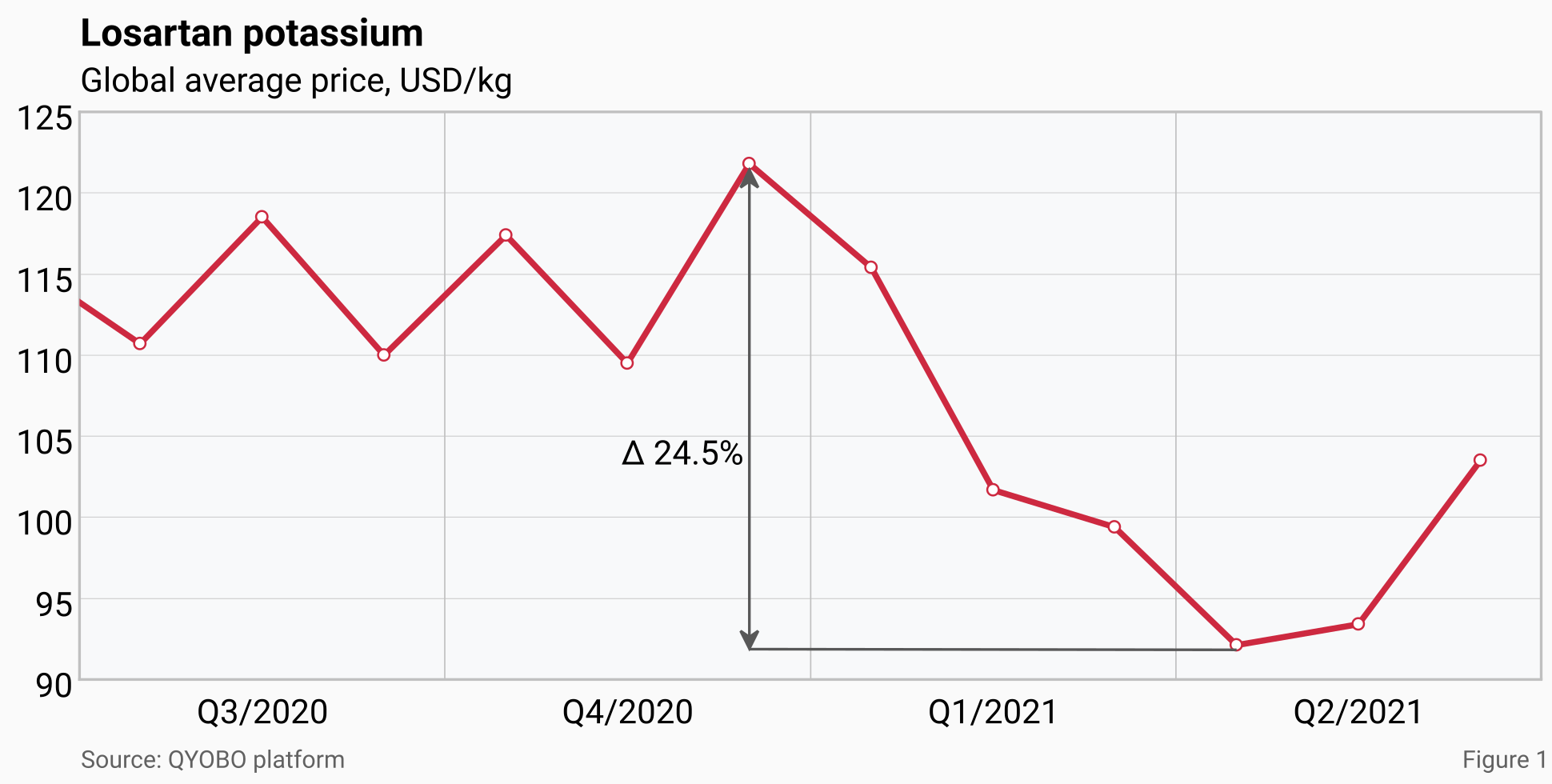
Global prices for the substance were fluctuating between 109.5 USD/kg and 121.8 USD/kg in the second half of 2020. During the following 4 months prices further decreased by 24.5% reaching a two-year low of 92.1 USD/kg in April 2021 (the all-time low of 53 USD/kg was reached in June 2017). This was followed by a 12.4 % price increase from April to June 2021.
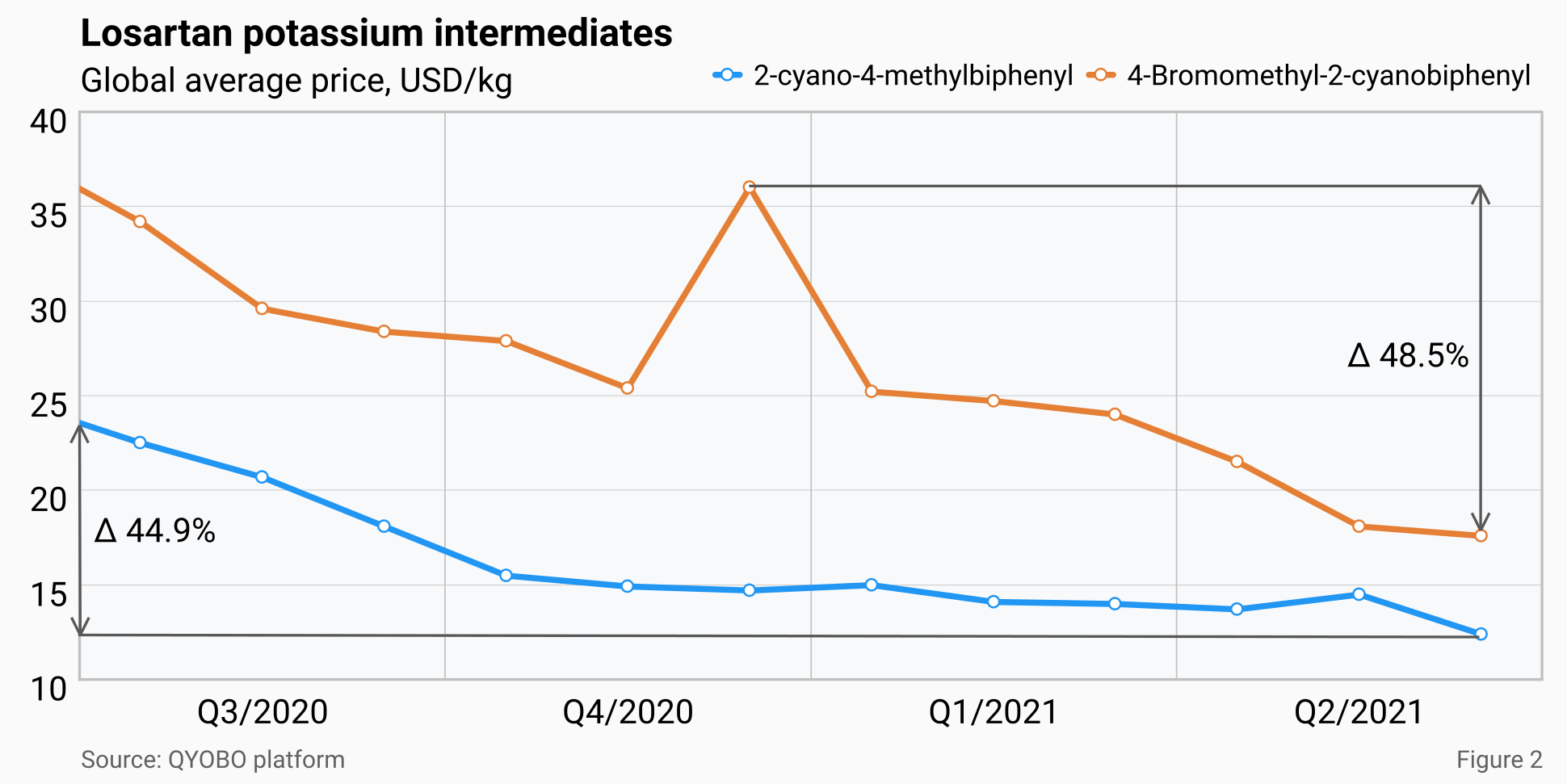
Global prices for both intermediates (KSM) of losartan potassium, 2-cyano-4-methylbiphenyl and 4-bromomethyl-2-cyanobiphenyl dropped by 44.9% and 48.5% respectively from July 2020 to June 2021. This price decrease was quite stable throughout the year with the exception of a temporary price spike of 36 USD/kg for 4-Bromomethyl-2-cyanobiphenyl in December 2020. (see figure 2)
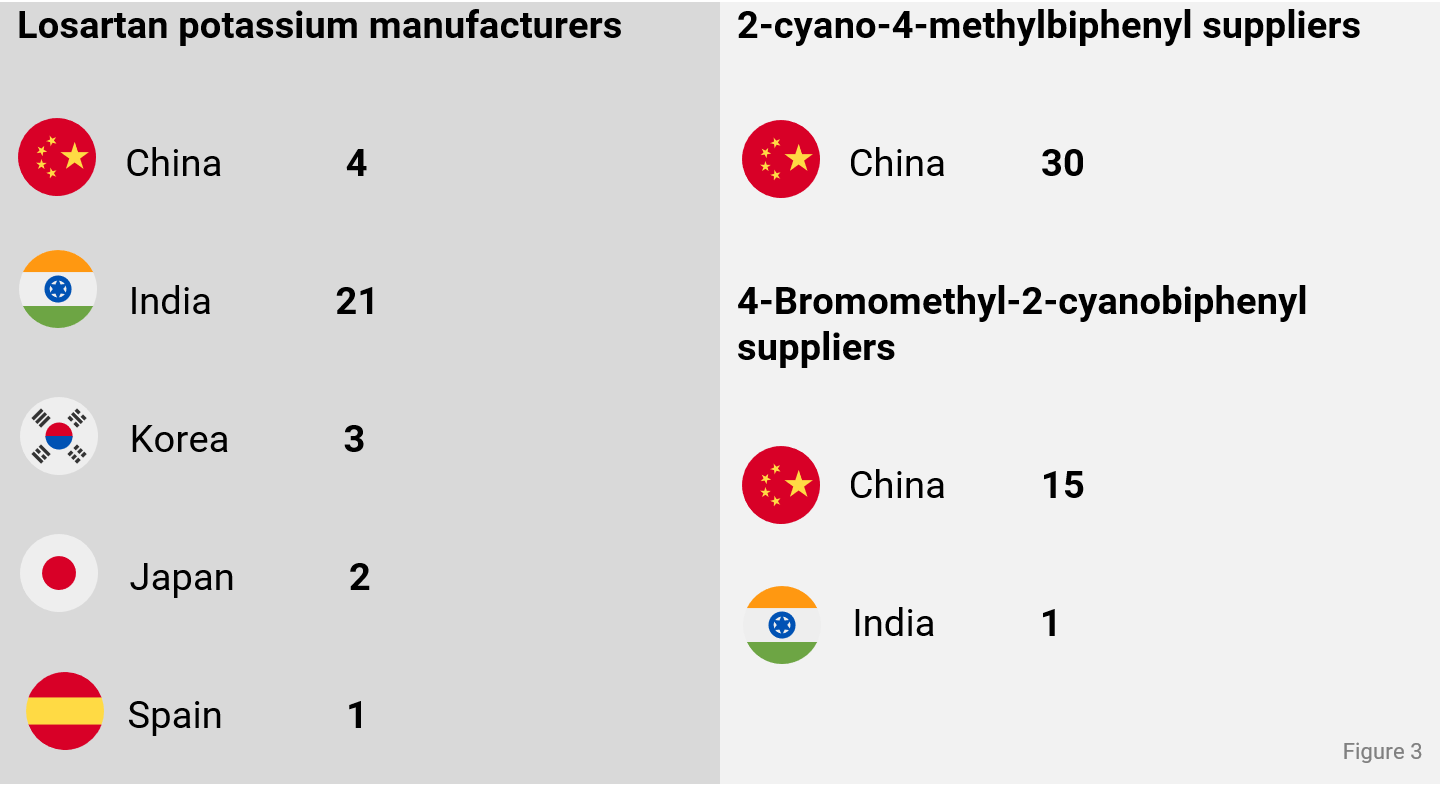 Taking a closer look into the manufacturing landscape of losartan potassium, manufacturers are located in 5 countries with a large majority in India. But with most of the suppliers for 2-cyano-4-methylbiphenyl and 4-bromomethyl-2-cyanobiphenyl located in China, the entire supply chain for losartan potassium is highly dependent on China as main manufacturing country. The QYOBO platform provides unique features to proactively identify and monitor risks along the supply chain starting from chemicals and intermediates to the API – feel free to reach out for a demo via our website or the contact details provided below.
Taking a closer look into the manufacturing landscape of losartan potassium, manufacturers are located in 5 countries with a large majority in India. But with most of the suppliers for 2-cyano-4-methylbiphenyl and 4-bromomethyl-2-cyanobiphenyl located in China, the entire supply chain for losartan potassium is highly dependent on China as main manufacturing country. The QYOBO platform provides unique features to proactively identify and monitor risks along the supply chain starting from chemicals and intermediates to the API – feel free to reach out for a demo via our website or the contact details provided below.
_
* e.g. the French Agence nationale de sécurité du médicament et des produits de santé (ANSM) recalling irbesartan hydrochlorothiazide, several drugs containing irbesartan and valsartan and irbesartan or Government of Canada recalling multiple batches of irbesartan, losartan and valsartan
Glossary
![]() U.S. Food and Drug Administration
U.S. Food and Drug Administration
About QYOBO GmbH
QYOBO’s mission is to improve access to essential medication for everyone by contributing to a more transparent, efficient and robust supply of pharmaceutical and chemical raw materials.
For this purpose, we’ve developed the QYOBO market analytics platform for APIs, intermediates and chemicals. From millions of trade, regulatory and financial datasets scattered around the world, our big data algorithms derive unique, actionable insights on market prices and trends, suggest suitable partners for your business and automate data-heavy workflows in procurement, supply chain and business development.
Founded in June 2019 and based in Munich, our company is pursuing its mission collaboratively with its international clients and has been recognized with numerous awards including the BASF market challenge and the Digital Innovation award 2020 by the German Federal Ministry for Economic Affairs & Energy (BMWi).
For further information please contact:
Ms. Thuy Linh Nguyen, Corporate Communications
communication@qyobo.com
www.qyobo.com
Disclaimer: The information in this article is not intended to be used for medication purposes. Please do not self-medicate and consult a physician/doctor for any questions with regard to your personal medical needs. This assessment has been prepared adhering to the highest quality standards based on a variety of external data sources (see sources) with the purpose of making distributed information accessible to a broader audience. The information contained in this document is provided on an “as is” basis and QYOBO GmbH assumes no responsibility or liability for the completeness, accuracy, usefulness or timeliness of the information provided. This article contains links to external websites operated by third parties upon which QYOBO GmbH has no influence. QYOBO GmbH does not assume any guarantee or liability for third party content.
² AEMPS, 04 August 2021.
Valsartan, Irbesartan STADA, varias presentaciones.
³ AEMPS, 04 August 2021.
Irbesartan Normon, varias presentaciones.
4 AEMPS, 04 August 2021.
Coaprovel y Karvezide, varias presentaciones.
5 Consalud, 05 August 2021.
Saltan todas las alarmas: Nueva retirada de fármacos con valsartán.
6 Healthcare Made Practical, 06 May 2021.
Blood pressure drug recalled.