API Market: Trends in Drug Master Filings Across the U.S., Europe, Japan, and China in 2025
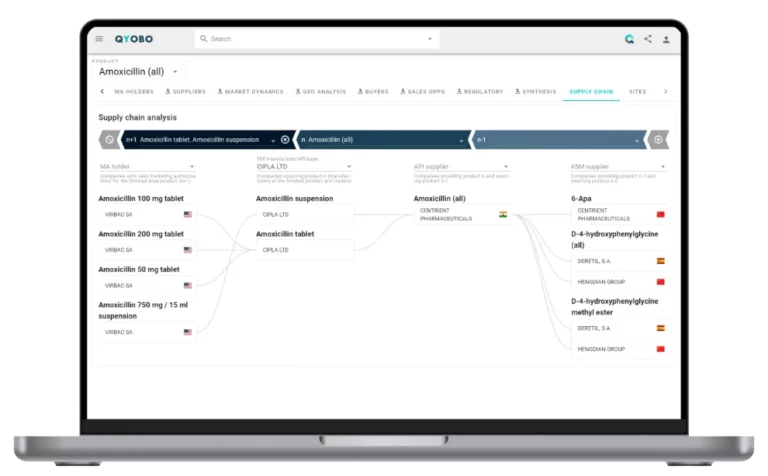
QYOBO’s intelligence platform is the world’s first to map the entire pharmaceutical manufacturing universe at site level. Drawing on QYOBO’s visibility into over 15,000 manufacturing sites worldwide, combined with Drug Master Filings (DMF) data from EDQM, NMPA, PMDA, and USFDA, this is the first analysis to compare API submissions across the U.S., Europe, Japan, and China.

Download Whitepaper for free
- First cross-market analysis of USDMF, CEP, JDMF, and CDMF data, powered by QYOBO’s unique end-to-end supply chain mapping.
- Filing trends and geographic insights into submission activity and the most frequently filed APIs in Q1 2025.
- Top companies by valid and new Drug Master Filings across major regulatory authorities.
free download
API Market trends with growth, leaders and innovation
Drop your email, and our QYOBO team will reach out to you shortly!
free download
Drug Master Filing Trends Across Key Markets (2025)
Drop your email, and our QYOBO team will reach out to you shortly!
By submitting this form, I declare that I have read and understood QYOBO’s privacy policy and give my explicit consent to the processing of my personal data for the stated purposes. I am aware that I can revoke my consent at any time.