Inspection Backlogs Post-COVID 2025
In the wake of COVID-19, global regulators are still working through substantial backlogs in pharmaceutical manufacturing site inspections. For the industry, these delays add uncertainty to supply chain planning, maintaining compliance, and anticipating regulatory measures. Using the QYOBO platform, we analysed more than 32,000 inspections and over 5,000 pharmaceutical manufacturing sites worldwide, revealing notable changes in the scale and frequency of regulatory oversight since 2020.

Download Whitepaper for free
- Global inspection backlogs and rising non-compliance rates since COVID.
- Financial and market risks from non-compliant inspection outcomes.
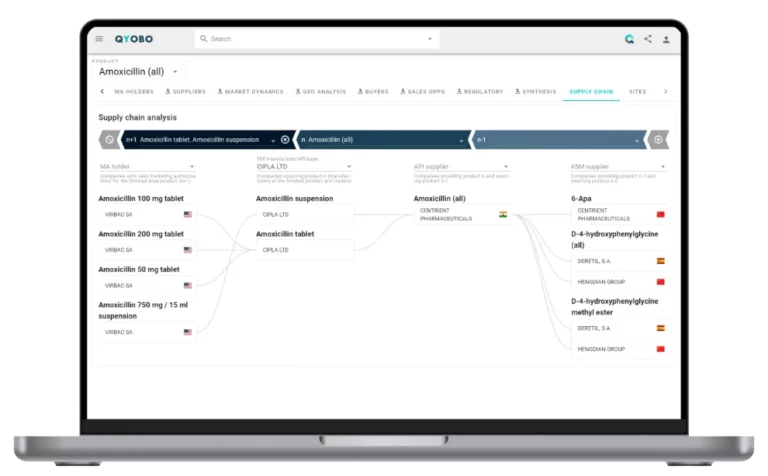
- QYOBO platform enables proactive oversight and risk monitoring across supply chains.
free download
API Market trends with growth, leaders and innovation
Drop your email, and our QYOBO team will reach out to you shortly!
free download
Inspection Backlogs Post-COVID 2025
Drop your email, and our QYOBO team will reach out to you shortly!
By submitting this form, I declare that I have read and understood QYOBO’s privacy policy and give my explicit consent to the processing of my personal data for the stated purposes. I am aware that I can revoke my consent at any time.